The Road to Regulatory Approval: Understanding FDA Special Review Designations
Valuation Services
Related
Never miss a thing.
Sign up to receive our insights newsletter.

Regulatory approval serves as an important differentiator of risk within the life science industry, but the many pathways for special review often compound an already complex system. As each pathway carries its own set of implications, intricacies and nuances, a holistic understanding is crucial for stakeholders across the spectrum as they navigate an evolving landscape. All too often, the U.S. Food and Drug Administration’s (FDA) special review pathways and the associated implications of each confuse and disorient passive, life science admirers and experts alike.
For starters, there are at least nine key special designations, most of which employ similarly optimistic wording, frequently connotating importance and speed. For instance, Accelerated Approval, Fast Track and Breakthrough designations offer little context or differentiation in their names alone, despite the significant variations in pathway sequence, degree of communication with the FDA, design of the clinical trials and economic incentives.
Furthermore, there is a great deal of information communicated through these special review designations, including estimated timeline for regulatory approval, relative capital requirements for clinical trials, potential therapeutic value and even market size, among others. These factors significantly influence life science executives’ risk assessment and strategic planning, including probability of successful/approval, capital requirements, and product pricing and volume upon reaching commercialization. Maintaining (and refreshing) a rudimentary understanding of these programs is important for not only maintaining life science literacy but comparing alternatives within the ever-growing life science landscape.
Given the critical, yet inaccessible nature of these special review designations, we provide an overview of the nine programs below and detail the differences in pathway for five of the major programs.
As a very cursory background, the role of the FDA is to evaluate a drug’s safety and efficacy based on data submitted in a New Drug Application (NDA) or Biologic License Application (BLA). Note: the difference between a drug and biologic being that drugs tend to be chemically synthesized, small-molecules, while biologics tend to be large, structurally complex molecules or mixtures that are not chemically manufactured. Notably, companies can, and often do, qualify for multiple special FDA designations.
| FDA Special Review Designations | |
|---|---|
| Orphan | Established in 1983, this designation focuses on the development of treatments for diseases that either affect fewer than 200,000 people in the U.S., or if affecting more, lack economic viability for drug development. Notably, the designation is granted based on the patient population, not the severity of the disease or the mechanism of action. (Note: 54% of the new drugs approved in 2022 held Orphan status) [1] |
| Priority Review | Established in 1992, this designation focuses on applications that demonstrate a “significant improvement in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions,” the determination of which falls under the FDA’s interpretation. Applications that qualify as a Qualified Infectious Disease Product or submitted with a Priority Review Voucher receive Priority Review status. (Note: 57% of the new drugs approved in 2022 held Priority Review status) [1] |
| Accelerated Approval | Established in 1992, this designation focuses on drugs treating serious conditions, offering significant advantages over existing therapies and demonstrating efficacy through surrogate endpoints. Unlike traditional clinical trial endpoints like overall survival or progression-free survival, surrogate endpoints predict clinical benefit more quickly. This program expedites treatment availability, especially for severe diseases with limited options. However, the FDA requires post-market studies to confirm surrogate results, which may be revoked if these studies fail to demonstrate efficacy or raise safety concerns. (Note: 16% of the new drugs approved in 2022 held Accelerated Approval status) [1] |
| Fast Track | Established in 1988, this designation focuses on drugs intended to treat serious conditions, with nonclinical or clinical data showing potential to address unmet medical needs. Unlike traditional submission, this program allows manufacturers to submit segments as data becomes available, enhancing efficiency, streamlining regulation and fostering collaboration between manufacturers and the FDA. Applications that qualify as a Qualified Infectious Disease Product or earn Breakthrough status also receive Fast Track status. (Note: 32% of the new drugs approved in 2022 held Fast Track Approval status) [1] |
| Breakthrough | Established in 2012, this designation focuses on drugs treating serious conditions, showing preliminary clinical evidence of substantial improvement over available therapies. Qualifying, which primarily treat severe or life-threatening conditions, often feature a novel mechanism of action compared to existing therapies for a particular disease. (Note: 35% of the new drugs approved in 2022 held Breakthrough status) [1] |
| Priority Review Vouchers | Established in 2007, this designation expands upon the Priority Review’s principles and further incentivizes drug development in underserved rare diseases, including rare pediatric diseases, tropical diseases, or Material Threat Medical Countermeasures. Notably, this voucher can be transferred to another drug or another manufacturer. |
| Regenerative Medicine Advanced Therapy (RMAT) | Established in 2016, this designation is akin to the Breakthrough but for certain innovative biologics, like cell and gene therapies and therapeutic human tissue-engineered products, reviewed by the FDA’s Center for Biologics Evaluation and Research (CBER). Thus, RMAT focuses on innovative biologics or advanced treatments, showing preliminary clinical evidence of substantial improvement over available therapies. Unlike the Breakthrough designation, RMAT eligible applications can qualify for Accelerated Approval based surrogate or intermediate endpoints. |
| Qualified Infectious Disease Product (QIDP) | Established in 2012, this designation is akin to the Orphan drug designation in that it encourages manufacturers to address unmet clinical needs through the development of new antibacterial and antifungal products. Qualifying drugs must target life-threatening bacterial or fungal infections or a resistant pathogen, as determined by the FDA. QIDP eligible products also qualify for Fast Track designations benefits as well as expanded market exclusivity. |
| Real-Time Oncology Review | Established in 2017, this designation focuses on straightforward oncology products with clear endpoints, offering a rapid decision from the FDA, well before the FDA’s official target decision date. Frequently, qualifying drugs utilize a mechanism of action familiar to the FDA, as opposed to more “cutting edge” technology. |
Adapted from [1] & [2].
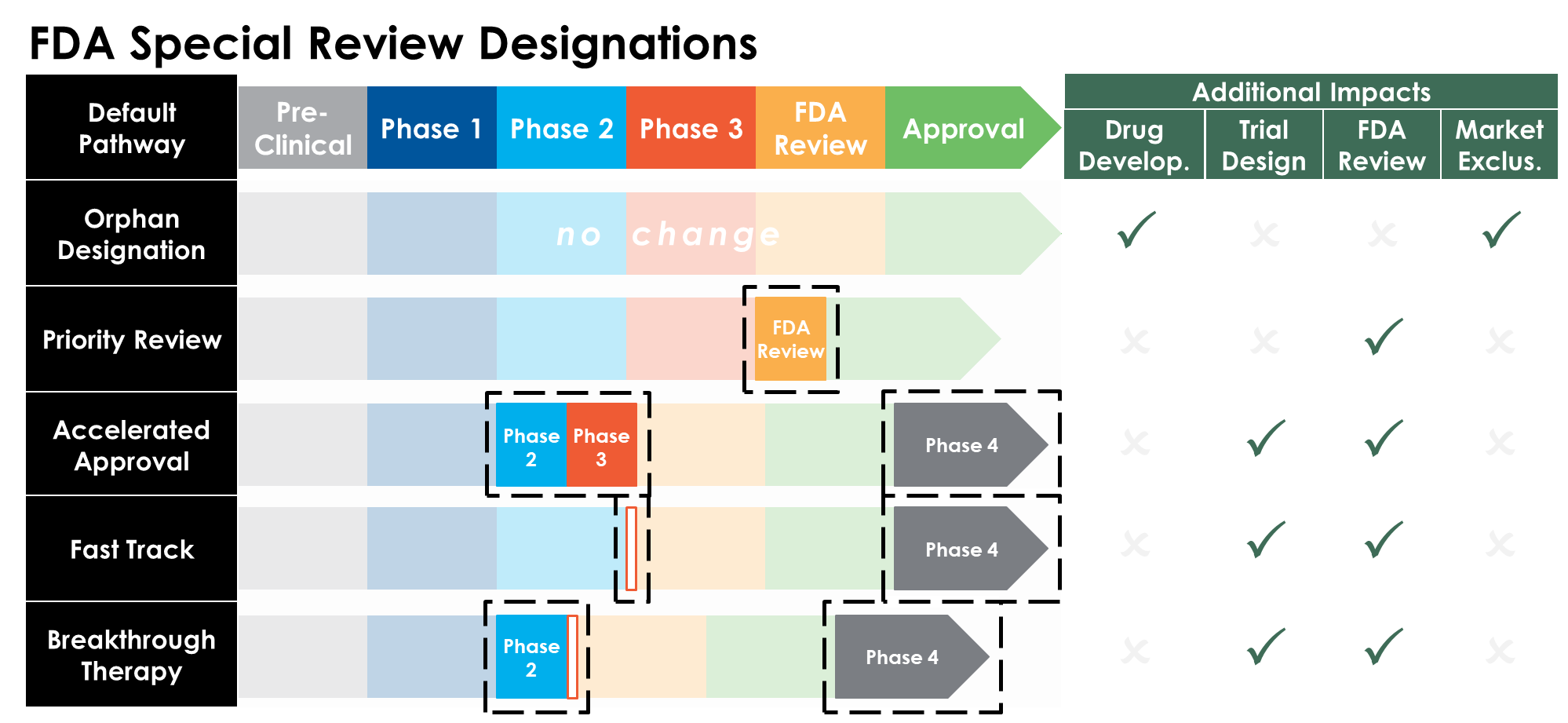
Adapted from [2].
These special review designations can drastically alter the timeline, capital requirements, and potentially the probability of successful approval, all of which impacts the overall regulatory risk assessment for the developer. There are notable economic incentives, including grants, tax incentives and expanded periods of market exclusivity, for which we will explore the valuation implications in greater detail.
Weaver empowers life science teams with holistic support to optimize their financial strategies and drive innovation. Contact us to learn more.
©2024
References
- Chandanais, R. (2023, September 8). An overview of the 9 FDA special designations for pipeline drugs. An Overview Of The 9 FDA Special Designations for Pipeline Drugs. https://www.outsourcedpharma.com/doc/an-overview-of-the-fda-special-designations-for-pipeline-drugs-0001
- Michaeli, D.T., Michaeli, T., Albers, S. et al.Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy. Eur J Health Econ (2023). https://doi.org/10.1007/s10198-023-01639-x
